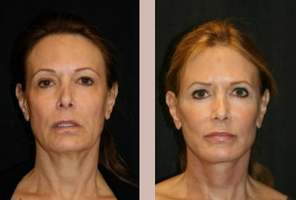
Photodynamic therapy, also known as blue light therapy, is a nonsurgical, minimally invasive outpatient procedure that is used in the treatment of several types of cancer, psoriasis, acne and sun damage. The therapy involves the use of a photo-sensitizing agent in conjunction with light of a specific wavelength and tissue oxygen.
FDA and Photodynamic Therapy
The US Food and Drug Administration (FDA) approved the use of photodynamic therapy in the treatment of severe acne in the year 2003. The administration also approved the use of the photo-sensitizing agent, Photofrin, for the treatment of esophageal cancer and non-small lung cancer. This agent relieves the symptoms of esophageal cancer and is helpful in treating cancers that don't respond to other treatments.
Photofrin was also approved for the treatment of pre-cancerous lesions in patients suffering from Barrett's esophagus. Other photo-sensitizing agents that have also been approved by the FDA are Levulan and Metvixia cream. Levulan is activated by blue light and has been approved for use on the face and scalp areas. It's used in the treatment of actinic keratosis and skin lymphomas such as mycosis fungoides. Metvixia is activated with red light and is used to treat non-hyperkeratotic actinic keratoses of the face and the scalp.