Skin Tyte treatments were given the green light from the FDA with the approval of the use of the laser used to perform the treatment. The treatment is done with a Polaris WR laser, manufactured by Syneron Medical, Ltd. The laser was approved for use in January 2004 and became a staple in dermatological offices all over the country. Check with your medical insurance provider as Skin Tyte is not covered by HMOs.
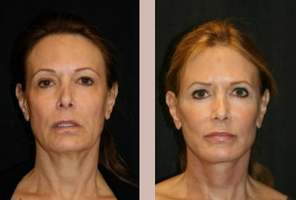
Skin Tyte is a laser treatment utilizing broad band light energy within the skin's dermis to tighten sagging skin and generally reduce the look of aging.
FDA Approval
While FDA approval was given to the overall process, it was the laser that had to undergo extensive testing before the laser and treatment procedure could be used. Extensive testing showed the Polaris WR laser to offer better results and a softer approach over the lasers and skin treatments used at the time.
Advantages to Choosing Skin Tyte
- Relatively painless compared to chemical treatments
- Shorter healing period over other forms of treatment
- You can immediately return to your daily routine with no downtime.
- The dermis is undamaged by the procedure, leaving better results.
- Polaris WR laser has a larger spot size and faster pulse, decreasing length of treatments.